图片
-
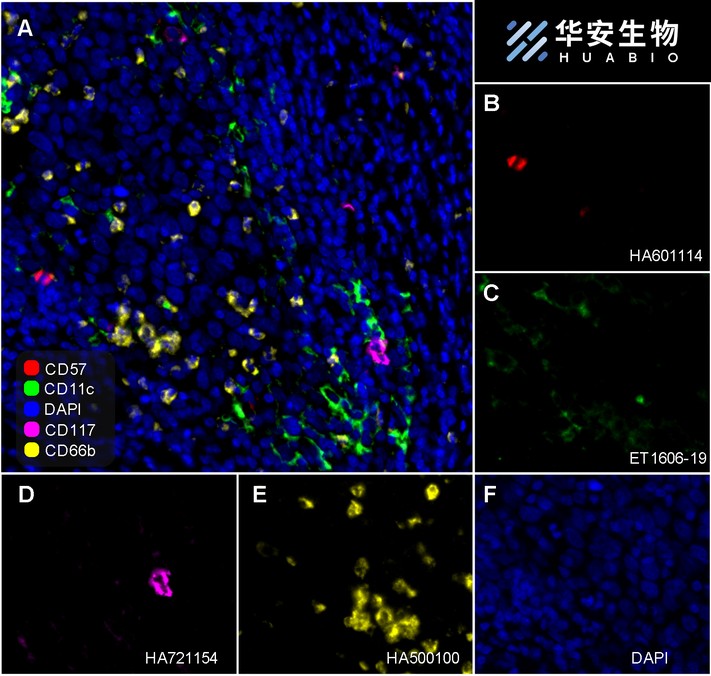
Fluorescence multiplex immunohistochemical analysis of the human cervical cancer (Formalin/PFA-fixed paraffin-embedded sections). Panel A: the merged image of anti-CD57 (HA601114, red), anti-CD11c (ET1606-19, green), anti-CD117 (HA21154, magenta) and anti-CD66b (HA500100, yellow) on human cervical cancer. Panel B: anti- CD57 stained on NKT cells. Panel C: anti-CD11c stained on dendritic cells. Panel D: anti-CD117 stained on mast cells. Panel E: anti-CD66b stained on neutrophils. HRP Conjugated UltraPolymer Goat Polyclonal Antibody HA1119/HA1120 was used as a secondary antibody. The immunostaining was performed with the Sequential Immuno-staining Kit (IRISKit™MH010101, www.luminiris.cn). The section was incubated in four rounds of staining: in the order of HA601114 (1/500 dilution), ET1606-19 (1/1,000 dilution), HA721154 (1/1,000 dilution), and HA500100 (1/1,000 dilution) for 20 mins at room temperature. Each round was followed by a separate fluorescent tyramide signal amplification system. Heat mediated antigen retrieval with Tris-EDTA buffer (pH 9.0) for 30 mins at 95℃. DAPI (blue) was used as a nuclear counter stain. Image acquisition was performed with Olympus VS200 Slide Scanner.
-
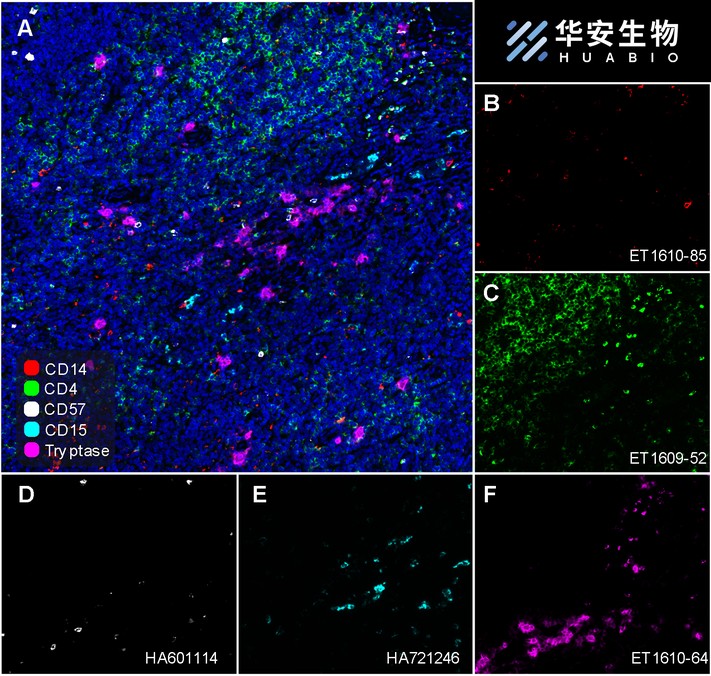
Fluorescence multiplex immunohistochemical analysis of Human tonsil (Formalin/PFA-fixed paraffin-embedded sections). Panel A: the merged image of anti-CD14 (ET1610-85, Red), anti-CD4 (ET1609-52, Green), anti-CD57 (HA601114, White), anti-CD15 (HA721246, Cyan)and anti-Tryptase (ET1610-64, Magenta) on tonsil. Panel B: anti- CD14 stained on monocytes. Panel C: anti-CD4 stained on helper T cells and Treg cells. Panel D: anti-CD57 stained on NK cells and T cells. Panel E: CD15 stained on granulocytes and monocytes. Panel F: anti-Tryptase stained on Mast cells. HRP Conjugated UltraPolymer Goat Polyclonal Antibody HA1119/HA1120 was used as a secondary antibody. The immunostaining was performed with the Sequential Immuno-staining Kit (IRISKit™MH010101, www.luminiris.cn). The section was incubated in five rounds of staining: in the order of ET1610-85 (1/800 dilution), ET1609-52 (1/800 dilution), HA601114 (1/1,000 dilution), HA721246 (1/500 dilution), and ET1610-64 (1/3,000 dilution) for 20 mins at room temperature. Each round was followed by a separate fluorescent tyramide signal amplification system. Heat mediated antigen retrieval with Tris-EDTA buffer (pH 9.0) for 30 mins at 95℃. DAPI (blue) was used as a nuclear counter stain. Image acquisition was performed with Olympus VS200 Slide Scanner.
-
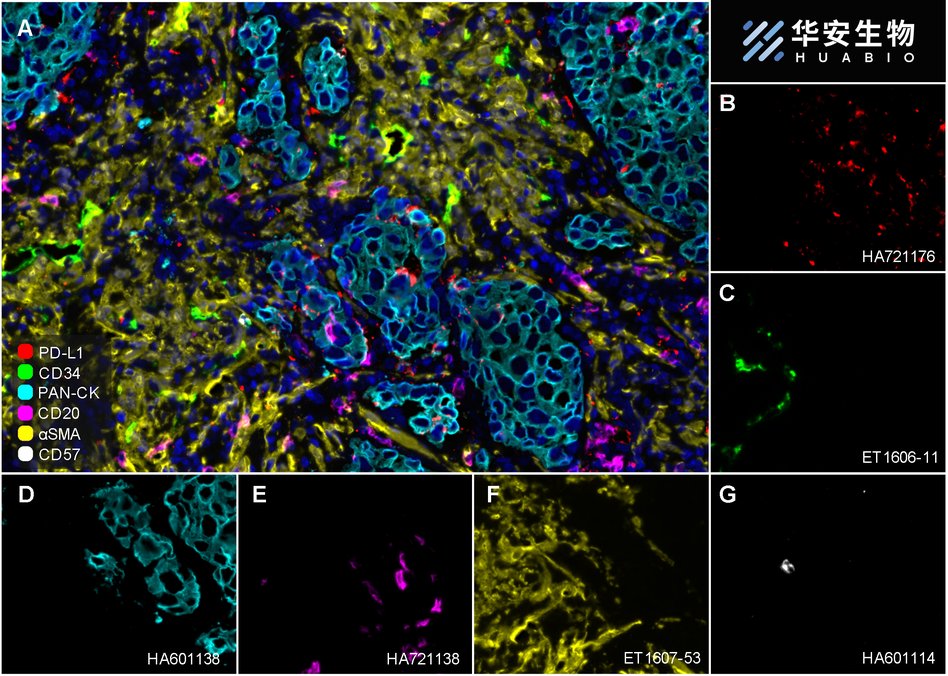
Fluorescence multiplex immunohistochemical analysis of Human non-small cell lung cancer (Formalin/PFA-fixed paraffin-embedded sections). Panel A: the merged image of anti-PD-L1 (HA721176, red), anti-CD34 (ET1606-11, green), anti-Pan-CK (HA601138, cyan), anti-CD20 (HA721138, magenta), anti-αSMA (ET1607-53, yellow) and anti-CD57 (HA601114, white) on NSCLC. Panel B: anti-PD-L1 stained on dendritic cells and macrophages cells. Panel C: anti- CD34 stained on endothelial cells. Panel D: anti-Pan-CK stained on cancer cells. Panel E: CD20 stained on B cells. Panel F: anti-αSMA stained on cancer-associated fibroblasts and smooth muscle cells. Panel G: anti-CD57 stained on NK cells and T cells. HRP Conjugated UltraPolymer Goat Polyclonal Antibody HA1119/HA1120 was used as a secondary antibody. The immunostaining was performed with the Sequential Immuno-staining Kit (IRISKit™MH010101, www.luminiris.cn). The section was incubated in six rounds of staining: in the order of HA721176 (1/1,000 dilution), ET1606-11 (1/1,000 dilution), HA601138 (1/3,000 dilution), HA721138 (1/2,000 dilution), ET1607-53 (1/3,000 dilution) and HA601114 (1/1,000 dilution) for 20 mins at room temperature. Each round was followed by a separate fluorescent tyramide signal amplification system. Heat mediated antigen retrieval with Tris-EDTA buffer (pH 9.0) for 30 mins at 95℃. DAPI (blue) was used as a nuclear counter stain. Image acquisition was performed with Olympus VS200 Slide Scanner.
-
Fluorescence multiplex immunohistochemical analysis of human tonsil (Formalin/PFA-fixed paraffin-embedded sections). Panel A: the merged image of anti-CD20 (HA721138, Cyan), anti-CD38 (HA721268, Violet) and anti-CD57 (HA601114, Yellow) on tonsil. HRP Conjugated UltraPolymer Goat Polyclonal Antibody HA1119/HA1120 was used as a secondary antibody. The immunostaining was performed with the Sequential Immuno-staining Kit (IRISKit™MH010101, www.luminiris.cn). The section was incubated in three rounds of staining: in the order of HA721138 (1/2,000 dilution), HA721268 (1/1,000 dilution) and HA601114 (1/1,000 dilution) for 20 mins at room temperature. Each round was followed by a separate fluorescent tyramide signal amplification system. Heat mediated antigen retrieval with Tris-EDTA buffer (pH 9.0) for 30 mins at 95℃. DAPI (blue) was used as a nuclear counter stain. Image acquisition was performed with Zeiss Observer 7 Inverted Fluorescence Microscope.
-
Immunohistochemical analysis of paraffin-embedded human Hodgkin's lymphoma tissue with Mouse anti-CD57 antibody (HA601114) at 1/50 dilution.
The section was pre-treated using heat mediated antigen retrieval with Tris-EDTA buffer (pH 9.0) for 20 minutes. The tissues were blocked in 1% BSA for 20 minutes at room temperature, washed with ddH2O and PBS, and then probed with the primary antibody (HA601114) at 1/50 dilution for 1 hour at room temperature. The detection was performed using an HRP conjugated compact polymer system. DAB was used as the chromogen. Tissues were counterstained with hematoxylin and mounted with DPX.
-
Immunohistochemical analysis of paraffin-embedded human tonsil tissue with Mouse anti-CD57 antibody (HA601114) at 1/200 dilution.
The section was pre-treated using heat mediated antigen retrieval with Tris-EDTA buffer (pH 9.0) for 20 minutes. The tissues were blocked in 1% BSA for 20 minutes at room temperature, washed with ddH2O and PBS, and then probed with the primary antibody (HA601114) at 1/200 dilution for 1 hour at room temperature. The detection was performed using an HRP conjugated compact polymer system. DAB was used as the chromogen. Tissues were counterstained with hematoxylin and mounted with DPX.
Please note: All products are "FOR RESEARCH USE ONLY AND ARE NOT INTENDED FOR DIAGNOSTIC OR THERAPEUTIC USE"








